Welcome to
OCRx website
Access its structure and content
OCRx What is it?

OCRx is an interoperability tool created by LabTNS researchers.
OCRx is developped through the integration of drug public data:
The Drug Product Database (DPD) maintained by Health Canada
The Canadian Clinical Drug Data Set (CCDD) maintained by Canada Health Infoway
This is a first version which is only focus on clinical drug description.
Packeging will be take into account in next release.
OCRx For what purpose?
Drug Identification Number (DIN), an eight-digit number, are generated for administrative purpose. The drug descriptions are mainly realise by the manufacturer (example for non-nconventional forms) and DIN code be modified for administrative reason (e.g., change in the name of the manufacturer).
The objective of OCRx is thus to:
To provide a standardized description for drugs allowing a non-ambiguous manipulation by human and computer
To improve the interoperability of drug-related information
To guarantee international compatibility by being compliant with IDMP principles
To improve drug entities recognition in free text
OCRx What is in it?
OCRx carries out a normalization of drug names
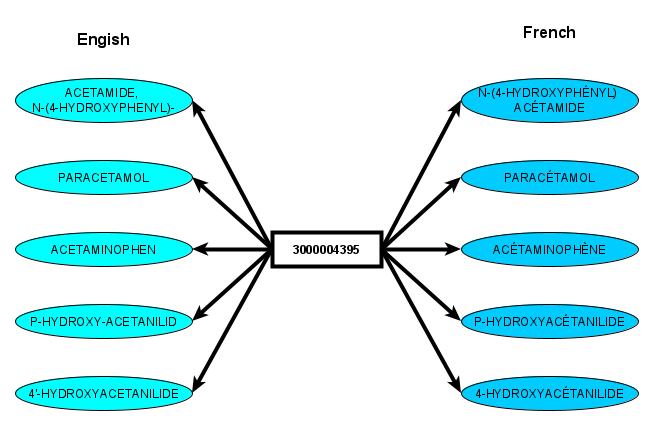
OCRx is based on a ontological structure that can be classified by usual reasoners (Fact++, Hermit or Pellet) in less than one seconde
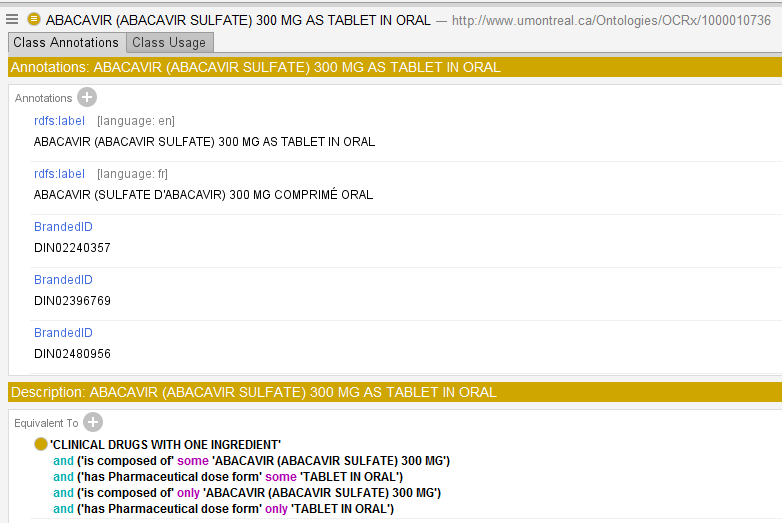
You can use our browser to explore OCRx content